
METTL12, Active (M332-30G)
FOR BULK ORDER REQUESTS PLEASE CONTACT US
Description :Recombinant full-length human METTL12 was expressed by baculovirus in Sf9 insect cells using an N-terminal GST tag.
Species :Human
Tag :GST tag
Expression System:Sf9 insect cells using baculovirus
Sequence :Full length
Genbank Number : NM_001043229
Specific Activity :Sample Kinase Activity Plot. For specific information on a given lot, see related technical data sheet.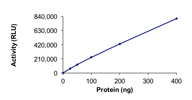
Purity :Sample Purity Data. For specific information on a given lot, see related technical data sheet.
Storage, Stability, and Shipping : Store product at –70oC. For optimal storage, aliquot target into smaller quantities after centrifugation and store at recommended temperature. For most favorable performance, avoid repeated handling and multiple freeze/thaw cycles.
Molecular Weight :MW 53 kDa.
Gene Aliases : CS-KMT; METTL12; U99HG
Scientific Background : Human methyltransferase like proteins (METTL) are part of a large protein family characterized by the presence of binding domains for S-adenosyl methionine, a co-substrate for methylation reactions (1). The methyltransferase-like protein 12 (METTL12) is localized in the mitochondrial matrix and methylates the lysine-368 that is localized near the active sites of citrate synthase (CS) (2). In response to alterations in metabolite levels, the METTL12-mediated methylation regulates the CS activity (3). Possible functions for the methylation of Lys-368 are in controlling substrate channeling itself, or in influencing protein–protein interactions in the metabolon (2).
References :
- Ignatova, V.V. et al: The interactome of a family of potential methyltransferases in HeLa cells. Scientific reports. 2019; 9.1: 1-9.
- Rhein, V.F. et al: Human METTL12 is a mitochondrial methyltransferase that modifies citrate synthase. FEBS letters. 2017; 591.12: 1641-1652.
- Malecki, J. et al: Uncovering human METTL12 as a mitochondrial methyltransferase that modulates citrate synthase activity through metabolite-sensitive lysine methylation. Journal of Biological Chemistry. 2017; 292.43: 17950-17962.
Product Sheets (By Lot #) :
